BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://jams.arakmu.ac.ir/article-1-6165-en.html
2- Department of Periodontics, Faculty of Dentistry, Arak University of Medical Sciences, Arak, Iran. ,
3- Department of Microbiology and Immunology, School of Medicine, Tuberculosis and Pediatrics Infectious Diseases Research Center, Arak University of Medical Sciences, Arak, Iran.
Introduction
With the addition of fluoride, the prevalence of dental caries has decreased, but many groups in the community still have dental caries [1]. Periodontal disease is one of the most common infectious diseases [4] and the spread of dental biofilm causes these diseases [8]. The compounds used in the treatment of periodontal disease usually have side effects, in addition to benefits [11]. Recently, plant compounds have been considered to reduce oral bacterial biofilms [12, 13]. One of these plants is peppermint whose effects on treatment of cold as well as antibacterial and antifungal properties have been reported. It has also been shown to have fewer side effects [14, 15]. Due to the importance and high prevalence of periodontal disease and the observed increase in cases of antibiotic resistance and side effects of overuse of chemical drugs, this study was conducted to investigate the possible antibacterial properties of peppermint on a number of microorganisms involved in periodontal disease.
Materials and Methods
The present study was a laboratory experiment. Fresh peppermint was prepared from the Medicinal Plants and Drugs Research Institute of Shahid Beheshti University, and was then traditionally washed several times with water. It was then dried in a dark place away from sunlight at a temperature of 24+2° C for a week and then milled. Alcoholic and aqueous extraction methods were performed. Various dilutions were prepared from the extract. Essential oil was extracted using Clevenger apparatus. Experiments were conducted on 4 standard bacteria purchased from the microbial bank of Iran, including Enterococcus Faecalis, Streptococcus Sanguinis, Eikenella corrodens, and Actinomyces Viscosus. The diffusion disc method was used for testing microbial susceptibility to the essential oil after microbial suspension preparation [14, 15].
Microdilution method was used to determine the microbial susceptibility, and culture method and MTT assay were used to determine the minimum bactericidal concentration. The analysis of the adhesion strength of biofilm in the microplate and the inhibitory effect on biofilm formation at different concentrations by optical density measurement were read by Elisa Reader. In order to perform the biofilm test, the biofilm was placed in triplicate and the amount of biofilm was obtained by calculating the average biofilm of these three wells and comparing it with negative control.
Results
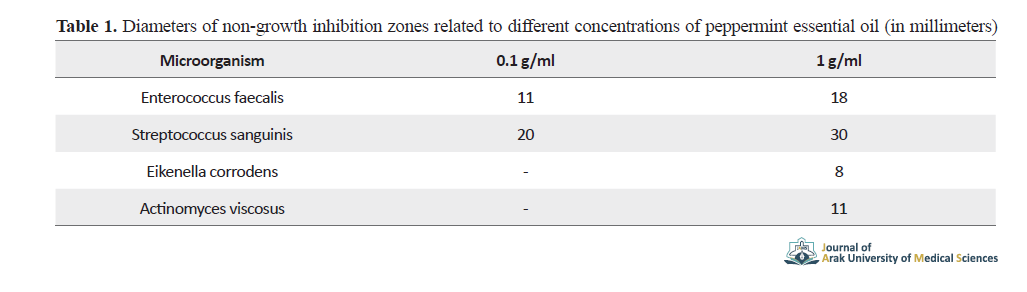
The diameters of non-growth inhibition zones related to different concentrations of peppermint essential oil (in millimeters) are shown in Table 1.
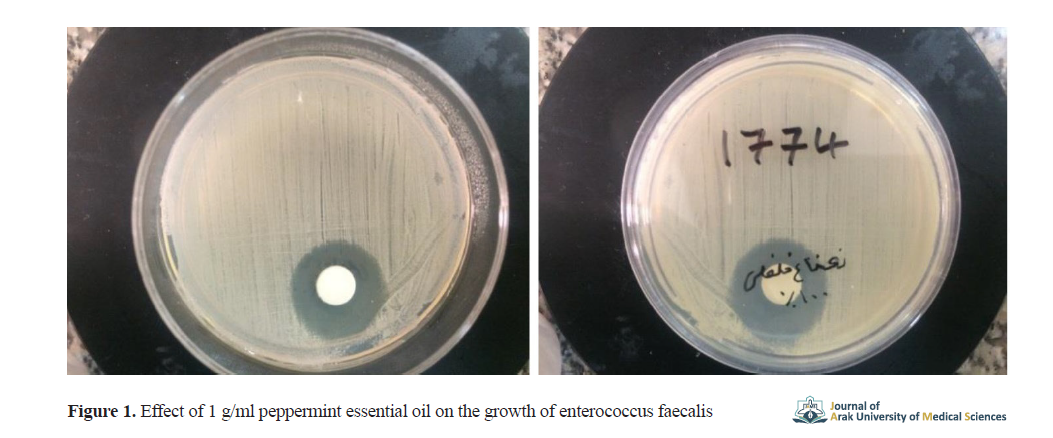
The 0.1 g/ml peppermint essential oil had an inhibitory effect on the growth of enterococcus faecalis and streptococcus sanguinis. Results showed the strongest effect of pure plant essential oil on streptococcus sanguinis and its weakest effect on eikenella corrodens. The effect of 1 g/ml peppermint essential oil on the growth of enterococcus faecalis is shown in Figure 1, as a sample.
Actinomyces viscosus did not grow up to well No. 5 (3.125 mg/ml concentration of essential oil), and the rest of the bacteria did not grow up to well No. 6 (1.562 mg / ml concentration of essential oil).
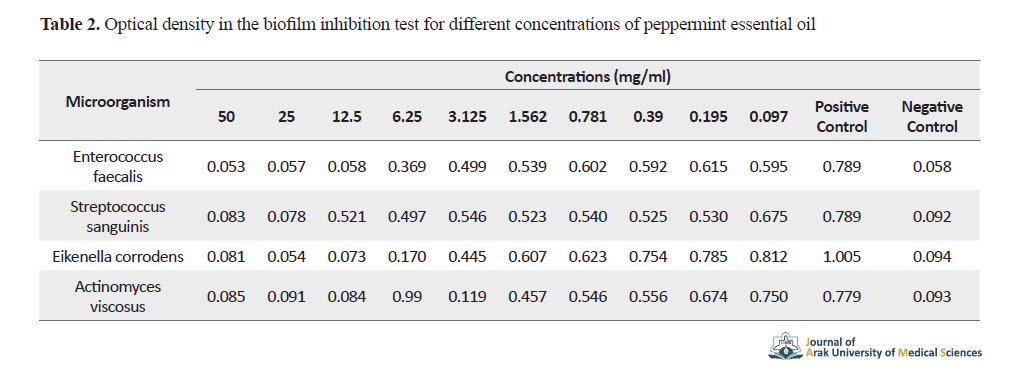
Actinomyces viscosus was inhibited to grow at concentrations ≥3.125 mg/ml and other bacteria at concentrations ≥1.562 mg/ml of peppermint essential oil. The optical density in the biofilm inhibition test for different concentrations of peppermint essential oil is shown in Table 2.
The bacterium actinomyces viscosus did not form biofilm up to well No. 4 (at concentrations ≥3.125 mg/ml) and other bacteria up to well No. 3 (at concentrations ≥12.5 mg/ml).
Discussion
Pure essential oil of peppermint showed the strongest inhibitory effect on streptococcus sanguinis followed by enterococcus faecalis, actinomyces viscosus and eikenella corrodens. The strongest bactericidal effect of peppermint essential oil was on eikenella corrodens followed by actinomyces viscosus, streptococcus sanguinis,and enterococcus faecalis. Moreover, its strongest inhibitory effect on the process of biofilm formation was observed in actinomyces viscosus followed by enterococcus faecalis, eikenella corrodens, and streptococcus sanguinis. In a study on the antibacterial effect of peppermint on the two strains of streptococcus sanguinis and Streptococcus mutans, the minimum inhibitory concentration and the minimum bactericidal concentration were investigated [16], and the results were consistent with the results of the present study.
In another study, the measurement of the non-growth inhibition zone diameters of Escherichia coli bacteria, aeromonas hydrophila, Staphylococcus aureus, and enterococcus faecium was performed after using peppermint essential oil [17] whose results were in agreement with our results. Another study showed that the combined extract of peppermint and mango plants reduced the bacterial adhesion of Streptococcus mitis and streptococcus sanguinis [19].
Conclusion
The limitations of the study were the low number of study strains, the difficulty of culturing some pathogens, and the lack of similar human studies. However, this study can be a guide for further studies in human simulated models and In Vivo studies. It was concluded that peppermint plant has antibacterial properties on the bacteria of enterococcus faecalis, streptococcus sanguinis, eikenella corrodens, and actinomyces viscosus, and also has inhibitory effect on them. Therefore, peppermint as a natural and effective antibacterial agent, has a potential application in the prevention of periodontal disease.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Arak University of Medical Sciences with code: IR.ARAKMU.REC.1397.15.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors met the writing standards based on the recommendations of the International Committee of Medical Journal Editors (ICMJE).
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Deputy for Research of Arak University of Medical Sciences for their financial support.
References
1.Heng C. Tooth decay is the most prevalent disease. Fed Pract. 2016; 33(10):31-3. [PMID] [PMCID]
2.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008; 46(4):1407-17. [DOI:10.1128/JCM.01410-07] [PMID] [PMCID]
3.Kim J, Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology. 2006; 94(1):10-21. [DOI:10.1007/s10266-006-0060-6] [PMID] [PMCID]
4.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017; 11(2):72-80. [PMID] [PMCID]
5.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005; 366(9499):1809-20. [DOI:10.1016/S0140-6736(05)67728-8]
6.Lovegrove JM. Dental plaque revisited: Bacteria associated with periodontal disease. J N Z Soc Periodontol. 2004; (87):7-21. [PMID]
7.Berger D, Rakhamimova A, Pollack A, Loewy Z. Oral biofilms: Development, control, and analysis. High-Throughput. 2018; 7:24. [DOI:10.20944/preprints201808.0174.v1]
8.Lasserre JF, Brecx MC, Toma S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials. 2018; 11(10):1802. [DOI:10.3390/ma11101802] [PMID] [PMCID]
9.Ouhayoun JP. Penetrating the plaque biofilm: Impact of essential oil mouthwash. J Clin Periodontol. 2003; 30(s5):10-2. [DOI:10.1034/j.1600-051X.30.s5.4.x] [PMID]
10.Jones SB, West NX, Nesmiyanov PP, Krylov SE, Klechkovskaya VV, Arkharova NA, et al. The antibacterial efficacy of a foam mouthwash and its ability to remove biofilms. BDJ Open. 2018; 4:17038. [DOI:10.1038/s41405-018-0005-5] [PMID] [PMCID]
11.da Costa LFNP, da Silva Furtado Amaral C, da Silva Barbirato D, Leão ATT, Fogacci MF. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc. 2017; 148(5):308-18. [DOI:10.1016/j.adaj.2017.01.021] [PMID]
12.Karim B, Bhaskar DJ, Agali C, Gupta D, Gupta RK, Jain A, et al. Effect of Aloe vera mouthwash on periodontal health: Triple blind randomized control trial. Oral Health Dent Manag. 2014; 13(1):14-9. [PMID]
13.Shoae Hassani A, Hamdi K, Ghaemi A. [In vitro Reduction in Colonization of Streptococcus mutans by honey beeswax ethyl acetate extract (Persian)]. J Arak Univ Med Sci. 2008; 11(4):87-95. http://jams.arakmu.ac.ir/article-1-290-en.html
14.Imai H, Osawa K, Yasuda H, Hamashima H, Arai T, Sasatsu M. Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios. 2001; 106 Suppl 1:31-9. [PMID]
15.Liang R, Xu Sh, Shoemaker CF, Li Y, Zhong F, Huang Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem. 2012; 60(30):7548-55. [DOI:10.1021/jf301129k] [PMID]
16.Shafiei Z, Haji Abdul Rahim Z, Philip K, Thurairajah N. Antibacterial and anti-adherence effects of a Plant Extract Mixture (PEM) and its individual constituent extracts (Psidium sp., Mangifera sp., and Mentha sp.) on single- and dual-species biofilms. PeerJ. 2016; 4:e2519. [DOI:10.7717/peerj.2519] [PMID] [PMCID]
17.Abdel-Hameed ESS, Salman MS, Fadl MA, Elkhateeb A, El-Awady MA. Chemical composition of hydrodistillation and solvent free microwave extraction of essential oils from Mentha piperita L. Growing in Taif, Kingdom of Saudi Arabia, and their anticancer and antimicrobial activity. Orient J Chem. 2018; 34(1):222-33. [DOI:10.13005/ojc/340125]
18.Shalayel MHF, Asaad AM, Qureshi MA, Elhussein AB. Anti-bacterial activity of peppermint (Mentha piperita) extracts against some emerging multi-drug resistant human bacterial pathogens. J Herb Med. 2017; 7:27-30. [DOI:10.1016/j.hermed.2016.08.003]
19.Wan Nordini Hasnor WI, Fathilah AR, Rahim ZHA. Plant extracts of Psidium guajava, Mangifera and Mentha sp. inhibit the growth of the population of single-species oral biofilm. Altern Integr Med. 2013; 2(1):1000102. [DOI:10.4172/2327-5162.1000102]
Received: 2019/10/13 | Accepted: 2020/02/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |